Ordinary Differential Equation Model Investigates the Dynamics of Legionnaires' Disease
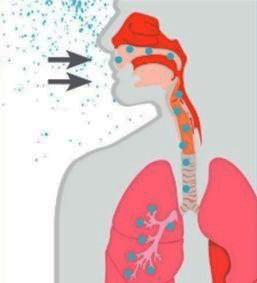
Legionnaires' disease is a type of pneumonia that is caused by bacteria of genus Legionella (most frequently, L. pneumophila). It typically spreads through airborne water droplets that enter the airways and eventually land in the lungs (see Figure 1), where they can cause serious infection and even lead to respiratory failure, shock and acute kidney and multi-organ failure, and brain sequelae. Possible sources of contamination include potable water for showers (especially in hotels and hospitals), cooling towers in large air conditioning systems, decorative fountains, and hot tubs. “The bacteria are everywhere,” Lihong Zhao, an incoming professor at Kennesaw State University, said. “It’s impossible to avoid and a little bit scary.”
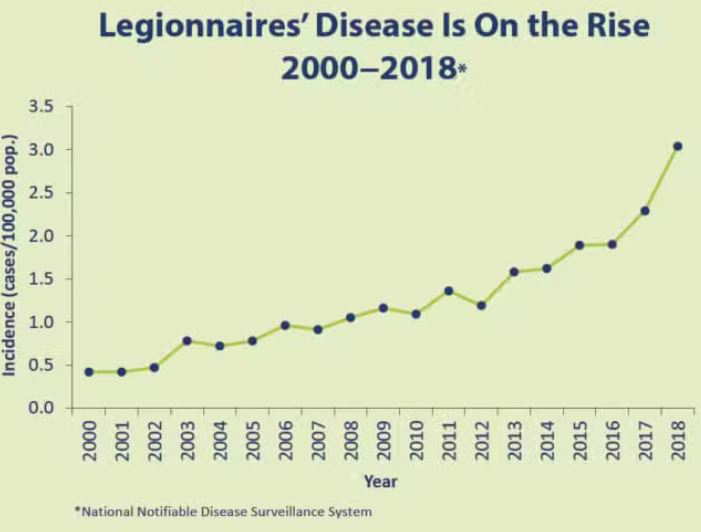
The first documented case of Legionnaires' in the U.S. appeared in a convention center in 1976. The disease burden has steadily increased since then, with a mortality rate of seven to 10 percent (see Figure 2). Yet despite this increase, overall knowledge of the condition remains relatively limited. It is also commonly underdiagnosed and underreported. Because it infects the lungs, Legionnaires' is usually detected via X-ray and treated with general antibiotics (rather than with a more invasive biopsy that would identify the incriminating bacteria and personalize treatment). Since no vaccine currently exists, authorities focus on prevention strategies that purify water resources to reduce the growth and spread of Legionella.
During a minisymposium presentation at the 2024 SIAM Annual Meeting, which is currently taking place in Spokane, Wash., Zhao utilized an ordinary differential equations (ODE)-based model to understand the dynamics of Legionnaires' disease. Specifically, she seeks to develop a mathematical modeling framework to better understand the transmission dynamics, examine the factors that contribute to the uptick in outbreaks, and ultimately generate mitigation strategies for government officials.
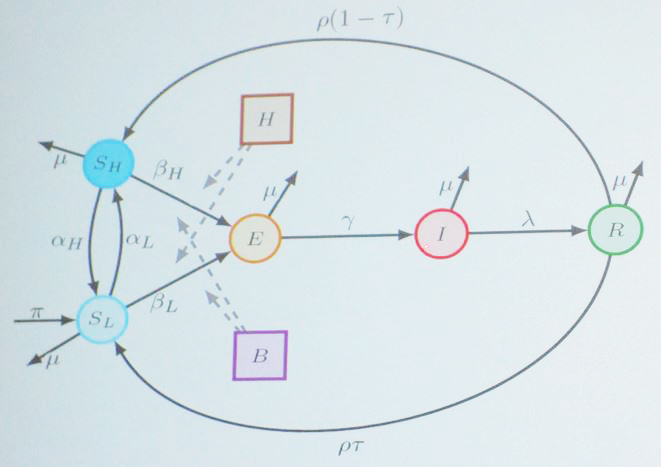
Zhao began with a modified susceptible-infected-recovered (SIR) model that categorizes people into the following compartments (see Figure 3):
- \(S_L\): susceptible individuals at low risk
- \(S_H\): susceptible individuals at high risk
- \(E\): exposed individuals
- \(I\): infected individuals (documented in the hospital)
- \(R\): recovered individuals
- \(H\): concentration of pathogens in the hospital
- \(B\): concentration of pathogens in the general environment.
As with any typical SIR model, individuals become exposed, infected, and then recovered; because immunity is not permanent, they eventually move back to \(S_H\) and \(S_L\). For the purposes of her study, Zhao made the baseline assumption that everyone within each compartment has the same probability of coming into contact with the pathogen.
Next, Zhao applied her toy model to existing data and used that data to infer the parameter values. The preliminary parameter estimation results did not perform as well as she had hoped, so Zhao examined potential risk factors and their possible influence on the model. Notably, not everyone who is exposed to Legionnaires’ disease actually gets sick. For example, individuals are more likely to develop the infection if they smoke, have a weakened immune system, suffer from a chronic lung disease or other serious condition, or are more than 50 years old.
Zhao used this information to modify her original SIR model and add a distinction between the hospital pathogens and those that simply exist within the environment. The updated model assumes that the hospital pathogens (\(H\)) only affect people with a higher probability of infection (\(S_H\)), while the pathogens in the environment (\(B\)) can still infect both \(S_L\) and \(S_H\).
Zhao concluded with a discussion about ongoing work to calibrate the model. Although her revised model performed better than the initial one, she is searching for more realistic assumptions about disease progression and additional datasets to infer other parameter values. Ultimately, Zhao wants to incorporate heterogeneity and human movement into her model — possibly in the form of partial differential equations rather than ODEs. Finally, she intends to explore health disparities in Legionnaires’ patients in the context of gender, race, economic stability, education, healthcare accesses, environment, and social constructs like stress and discrimination.
About the Author
Lina Sorg
Managing Editor, SIAM News
Lina Sorg is the managing editor of SIAM News.